
An occipital nerve stimulator (ONS) is a form of neuromodulation therapy that is designed to treat craniofacial pain and headache. Find out more about the therapy here.
The occipital nerve stimulator (ONS) procedure comprises an implantable device composed of a pulse generator and an electrode. The lead of this device is placed into the subcutaneous tissues innervated by the lesser and greater occipital nerves. Then the pulse generator is also implanted into a subcutaneous pocket of the patient’s abdomen, chest, or back.
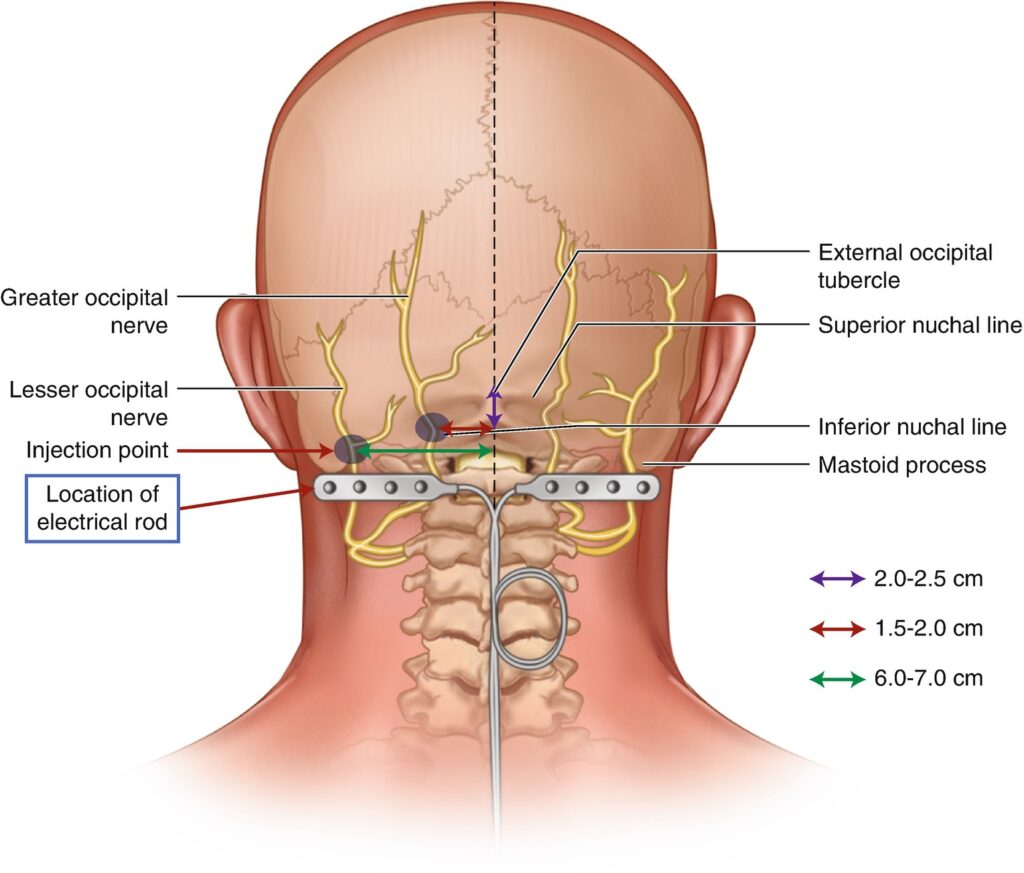
In the occipital nerve stimulation procedure, the doctor inserts a small device at the base of the skull where the occipital nerves originate. The device has leads linked to a pulse generator that generates electrical impulses to the occipital nerve.
The pulse generator is often implanted under the collarbone (clavicle), buttock (gluteal) or abdominal areas.
Occipital nerve stimulation in primary headache syndromes are as follows:
Many painful medical conditions can be effectively treated by performing occipital nerve stimulation. Following are some conditions, where standard medical therapies are of limited help:
It is important to inform the surgeon in case of the following:
Post-surgery, the wound on the chest is usually covered with a sterile dressing. The patient is advised to keep this wound covered for at least 3 days after the occipital nerve stimulation procedure. The back of the patient’s head will not be covered in any dressing as it does not stick to the hair properly.
The patient is allowed to take shower the next day after surgery. They should remove the dressing and then after a shower replace it with a new one.
Inspection of wounds is required to be done daily. This might need assistance to check the wounds at the back of the head.
If the patient notices any of the following, then it is important to call the doctor immediately:
Occipital nerve stimulation surgical procedure is generally effective and safe. However, there are some risks associated with this procedure, like any medical procedure. The common side effect is irritation or pain at the site of injection. Some related side effects that a patient experiences after receiving an injection is mentioned as follows:
In the case where the electrodes have moved from their position, a surgical review of the placement of the wires or electrodes may be required.
According to a study, 23 out of 25 patients who underwent an ONS trial, went on to have a permanent implantation procedure of ONS. Nine of the refractory chronic migraine (RCM) patients reported a decrease of more than 50% headache pain intensity and/or frequency in long-term follow-up i.e., 11–77 months. Patients reported more than 50% reduction in pain intensity and/or frequency at 28–31 months.
Various evidence and studies conducted depicts that patients with an occipital nerve stimulator had improved quality of life. 67% of patients from the active group showed improvement in the quality of life, as opposed to 17.2% of patients from the control group. Most patients in the active group were satisfied i.e., 51.4%, while 19.2% in the control group were satisfied.
According to a study presented at the 15th Annual Meeting of the North American Neuromodulation Society, patients who received 12 weeks of occipital nerve stimulation therapy informed significantly less pain, fewer headache days, and better quality of life than controls did.
The exact time of alleviating pain after the occipital nerve stimulator usually varies from one individual to the other. However, it can help in relieving pain for months in some individuals.
It is recommended mostly when medications and other related treatment options do not provide any relief. Or their side effects are too severe, in such cases the surgery may be of value.
In selected conditions as mentioned above, after the occipital nerve surgery is performed, around 60% of candidates can experience total elimination of headaches. While 80% to 90% of the remaining patients may have a 50% reduction in frequency and severity of the headaches.
The battery or the electrode life depends on the total hours of use in a day or the amplitude of the device. Typically, a rechargeable battery requires replacement after around 7 to 9 years. While a lithium battery persists for 3 to 5 years without any requirement to change.
MRI scans are routine procedures and are relatively safe. However they can sometimes interfere with the treatment delivered to the patient. The working of the neurostimulator devices, may result in injury. Before getting an MRI scan done with an implanted device, it is important for the patient to make sure that device is eligible to accept that particular scan.
Some other kinds of commonly used devices may also interfere with the neurostimulator functioning. It is still advisable to take particular caution when the person with such stimulator get close to security systems. It is therefore, important to ask for assistance to bypass the system. These systems may include the following:
If it is mandatory to pass through one of these devices, then the patient should turn off the nerve stimulator and proceed with caution. Once the patient has passed through such detectors, it is important to check the status of the nerve stimulator.
Surgery is usually performed by a neurosurgeon or brain spine and pain specialist. A skilled anaesthetist along with a surgical assistant will be present during the whole procedure.
The patient probably needs some repeated programming alterations that may include pain medications, in the initial few months to optimise their pain relief. These pain medications can be decreased as the pain is tolerated.
Potential outcomes of not treating your condition include: